|
Hok-E-News, Virginia Tech Magazine's online-only feature, is updated quarterly.
 University to develop climate commitment, sustainability plan University to develop climate commitment, sustainability plan
by Mark Owczarski
|
|
Virginia Tech President Charles W. Steger has announced that the university will develop a campus sustainability plan--aimed at reducing global warming emissions in everyday campus operations--by the end of the 2009 spring semester.
To achieve this goal, the latest step in ongoing sustainability efforts by the university, Steger has charged the recently formed Committee on Energy and Sustainability to develop the "Virginia Tech Climate Action Commitment."
"Virginia Tech will be better served by developing a sustainability plan that is specific to our university community," said Steger. "This plan will have a significant impact on our policies, operations, and the budget of the university. In order for this initiative to be successful, I believe we need to secure support of the entire university community.
|
|
|
|
|
|
|
The plan will be developed in close collaboration with the Office of the Vice President for Administrative Services, he said, and will include clear, measurable, and realistic goals.
"Campus sustainability has strong support among university leadership, and I believe Virginia Tech will become a leader in campus sustainability issues."
The university has initiated several sustainability projects in recent years. A complete list of those initiatives can be found on the university's sustainability website.
The development of the Virginia Tech Climate Action Commitment, which will be pursued in lieu of signing the generic President's Climate Commitment, will be submitted to Steger and the Commission on University Support by the end of the fall 2008 semester. Once approved by the commission, the draft plan will be submitted to University Council, with the goal of formal adoption of the plan by the end of the 2009 spring semester.
A subcommittee of the Committee on Energy and Sustainability, chaired by John Randolph, professor and director of the School of Public and International Affairs in the College of Architecture and Urban Studies, will meet throughout the summer to develop a draft plan.
In addition to Randolph, members of the Committee on Energy and Sustainability are Denny Cochrane, energy and sustainability coordinator, Office of the Associate Vice President for Facilities; Mike Coleman, associate vice president for facilities; Jack Davis, professor and dean, College of Architecture and Urban Studies; Angella DeSoto, undergraduate student, College of Architecture and Urban Studies; Michael Ermann, associate professor of architecture, College of Architecture and Urban Studies; Bruce Ferguson, assistant director for university planning, design, and construction, Department of Facilities; Natalya Hallanan, undergraduate student, College of Architecture and Urban Studies; Richard Hirsh, professor of history, College of Liberal Arts and Human Sciences; Scott Hurst, university architect; Rick Johnson, director of housing and dining services; Jack Lesko, professor of engineering science and mechanics and special assistant for energy initiatives, Office of the Vice President for Research; Rob Lowe, environmental engineer, Department of Environmental Health and Safety Services; Steve Mouras, director of transportation, Department of Facilities; Erik Olsen, assistant coordinator, Virginia Tech Recycling; Brian Perkins, graduate student in the College of Natural Resources; Mary Seyler, senior buyer, Purchasing Department; and Tom Tucker, architectural planner and vice president of the Staff Senate.
|
 Virginia Tech and Georgetown University to offer joint graduate degree in biomedical technology development and management Virginia Tech and Georgetown University to offer joint graduate degree in biomedical technology development and management
by Barbara L. Micale |
|
|
|
Classes will begin in fall 2008 for the new master of science in biomedical technology development and management, a joint graduate level degree created by Virginia Tech and Georgetown University.
The degree program was created in response to future directions in medical product discovery and development and the emerging needs of industry and regulatory agencies. Applications are available online.
Curriculum for the degree program integrates science with technology, management, ethics, and public policy and draws on the strengths of Virginia Tech in science, industrial and systems engineering, and business and management and Georgetown's medical research program. Students may apply to and matriculate at Virginia Tech or Georgetown. But, in either case, the instruction, tuition, and fees are identical, and the degree will be jointly conferred from both universities.
The master of science in biomedical technology development and management requires 30 semester hours. Each degree candidate must complete and successfully defend a research paper or a project and report demonstrating in-depth knowledge of a particular topic and the ability to analyze information, think critically, and communicate effectively.
"Working with other universities in the Washington, D.C., metropolitan area is an integral component in our goal to increase research in the National Capital Region," said Jim Bohland, vice president and executive director of Virginia Tech National Capital Region Operations. "We have successfully collaborated with Georgetown in the past, both in research projects and with academic programs designed for graduate students from the Food and Drug Administration. We welcome this opportunity to continue and expand our partnership."
The focus of the degree program is to develop professionals proficient in a variety of analytical tools, including modeling and simulation for business decision making. The program will utilize a cross-disciplinary approach covering drug development, medical devices, clinical investigation, regulatory affairs, and pharmaceutical quality assurance to address the industry-recognized need to produce well-rounded professionals conversant in multiple areas.
"The most compelling evidence of demand for graduates with this degree is that the Food and Drug Administration's Center for Devices and Radiological Health has contracted with Virginia Tech and Georgetown University for its current employees to take courses included in the new program," said Bohland.
|
|
 Smart brake light system would provide more information to drivers Smart brake light system would provide more information to drivers
by Susan Trulove
|
|
| You are driving in heavy traffic. The brake lights on the car in front of you come on. Is the car slowing or is it going to stop? It slows to 25 mph and the lights go off. You drop back. The car in front of you stops suddenly! You stop just in time. The car behind you collects your rear bumper.
"The problem is that brake lights are yes and no, on and off," according to John Hennage of Montross, Va., a Ph.D. mechanical engineering student in Virginia Tech's College of Engineering. "The driver behind does not know the speed at which the car in front is slowing or stopping. The only other signal would be the smoke off the tires."
The solution is an intelligent brake light system that communicates slowing and urgent stopping, rather than simply that the brake pedal is being touched.
|
|
|
|
|
|
|
"A driver could be tapping his foot in time to music, and the brake lights would blink. Or, a driver can rest her foot on the pedal, and the lights would glow. It's not enough information for the following driver," said Hennage.
With the support of Manassas, Va., businessman Meade Gwinn, Hennage and Virginia Tech mechanical engineering Professor Mehdi Ahmadian have invented an intelligent brake light system, which they showed at the Mid-America Trucking Show at the Kentucky Fair and Exposition Center in Louisville in March.
Gwinn came up with the idea for communicating braking speed after being rear-ended on Rt. 66 in Northern Virginia. "It was part of a chain-reaction accident," he said. Afterward, he walked down the line of cars to make sure others were okay. "Two cars back was a young woman with a child in the car. They were okay, but she kept saying, 'I couldn't tell how fast he was stopping.' I thought, wouldn't it be a good idea if rear tail lights communicated better and the following driver knew how fast you were stopping so they could take appropriate action?"
Years later, his youngest daughter, a student at Virginia Tech, suggested Gwinn try and get in touch with one of the engineering departments at the university. In 2000, Gwinn wrote to the university president, which led to a meeting with Walter O'Brien, professor and then head of the mechanical engineering department. "He was very helpful and encouraging, saying that this concept had the potential of great application at a very low cost," Gwinn said. "He subsequently introduced me to Dr. Mehdi Ahmadian, who was able to develop this project into a teaching/research curriculum over the next several years."
Ahmadian contacted Hennage to help the group of students who were assigned the problem. "I know electricity and had experience programming microcontrollers," said Hennage, who had previously developed LED lights for commercial trucks, which Ahmadian knew.
The students developed a horizontal light bar. Lights in the middle glow amber for slowing. When stopping speed crosses a threshold to urgent, red lights flash on either side of the amber lights. If deceleration is rapid, all of the lights flash red.
"The draw backs are that the light bar would be an additional brake light because the law forbids altering original equipment," said John Talerico, a licensing associate with Virginia Tech Intellectual Properties Inc. (VTIP). But the biggest obstacle is that the light bars cost $50 each to produce."
In fall of 2007, Ahmadian and Talerico approached Hennage about developing a cheaper unit that does the same thing by tapping into existing lights. "It would be for commercial trucks rather than private cars because commercial vehicles typically have redundant lights," Hennage said. "Private cars are 10 to 15 years behind commercial vehicles in terms of LED lighting."
Hennage developed a gravity or deceleration sensor control. Under normal braking--to slow or to stop slowly--the tail lights work in the normal fashion. But under heavy braking, extra lights flash.
"We also have the ability to connect other sensors to the microcontroller, such as from the automatic braking system, the automatic traction control, and the collision avoidance system," said Hennage. "If any of these systems are activated, lights could flash to alert drivers of nearby vehicles."
"There are various ways for this invention to work and we have a working prototype," said Talerico. "A manufacturer can take the specifications and produce this circuit in mass quantities."
Gwinn said, "Not only is this concept approaching potential commercialization, which will be most gratifying, but the educational benefit derived by numerous mechanical engineering students over the years is very heartwarming to me. I have met so many talented and enthusiastic students to have made significant contributions to the concept.
"The real reward to all of us, however, is to know that if this venture works out, millions of drivers will find the roads a much safer place to drive," said Gwinn. "In the end, we are all winners!"
For more information, contact Talerico at jtalerico@vtip.org or (540) 951-9376.
|
 Nanoscience will change the way we think about the world Nanoscience will change the way we think about the world
by Susan Trulove
|
|
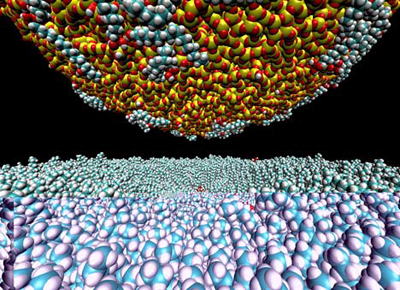 |
|
The ubiquity of mineral nanoparticles in natural waters, the atmosphere, and in soils and their intriguing properties provide Earth scientists with another dimension in which to understand our planet.
So states a team of scientists from seven universities in a review article in the March 21, 2008, issue of Science, "Nanominerals, Mineral Nanoparticles, and Earth Chemistry."
The way minerals influence earth is more complex than previously thought. Physical, chemical, and biological processes on Earth are either influenced or driven by the physical and chemical properties of minerals, of which 4,500 species have been described. Minerals have an enormous range of physical and chemical properties due to a wide range of composition and structure, including particle size.
|
|
|
|
|
When the National Science Foundation (NSF) wanted expert opinion on the important questions that need to be addressed in order to advance the understanding of nanoparticles in the environment, Michael Hochella Jr. was contacted to assemble a group of "cutting-edge young scientists with new ideas," he said. University Distinguished Professor of Geosciences at Virginia Tech Hochella is a pioneer in the field whose research is funded by the NSF, among others. "While we were together, we thought, why not write our study and submit it to Science," Hochella said.
"The article looks at the field, where it's come from, where it's going, and how it is going to change the way we think about geoscience and the world," he said. A perspective article is a great challenge to write, considering Science's limits on length and number of citations, he added.
The authors are Hochella; his former Ph.D. students Steven K. Lower, now a professor in the School of Earth Sciences and School of Environment and Natural Resources at the Ohio State University, and Patricia A. Maurice, now professor of civil engineering and geological sciences and director of the Center for Environmental Sciences and Technology at the University of Notre Dame; along with R. Lee Penn assistant professor of chemistry at the University of Minnesota; Nita Sahai, associate professor of geology and geophysics at the University of Wisconsin-Madison; Donald L. Sparks, chair of plant and soil sciences and professor in three departments at the University of Delaware; and Benjamin S. Twining, assistant professor of chemistry and biochemistry at the University of South Carolina.
Minerals, it is generally agreed, are naturally occurring crystalline substances having a characteristic and defined chemical composition. Each mineral expresses a set of specific physical and chemical properties. In addition, nanominerals have one critical difference. They express a range of physical and chemical properties depending on their size and shape.
"This difference changes our view of the diversity and complexity of minerals and how they influence Earth systems," Hochella said.
Where nanominerals are located
Nanominerals are widely distributed throughout the atmosphere, oceans, surface and ground waters, soils, and in most living organisms and even within proteins.
Oceans may be the principal reservoir, since they cover 70 percent of the Earth's surface. There, nanominerals can come from processes associated with both living and non-living things, Hochella said. "Every mineral goes through a nanophase stage as it begins to grow. If they begin to grow at many sites but don't continue to grow much after they form, you will end up with a lot of them and they may persist."
In addition to growth and weathering, mineral nanoparticles can be generated from mechanical grinding. One of the most interesting and important places where this happens is along earthquake-generating faults in the Earth's crust, reported by several researchers cited in the review.
There is a distinction between clusters of atoms and nanoparticles, Hochella said. "The difference seems to be that clusters start to approach the size of the smallest nanoparticles, but the atoms in many of these small clusters are not packed very tightly together. They are not dense. The nanoparticles represent a much denser packing of atoms, more like a real mineral, or at least approaching the atomic packing density of a larger mineral."
The essence of nanoscience is observing, measuring, and understanding the variations of properties and reactivities as a function of size and shape. Structural variations that respond to size change or surface-area change may include expansion and contraction of bonds, changes in bond angles, and variations in population and distribution of vacancies and other defects such as steps, kinks, edges, and corners. In the smallest nanoparticles, this results in a redistribution of electronic structure that affects reaction characteristics with the outside world. Measurement of these aspects remains a great challenge and priority for future mineralogists, the authors note.
The size at which properties and reactivities change can be measured and depends upon the mineral, whether it is a metal, semiconductor, or insulator; and on the property being measured, whether optical, mechanical, or electrical.
Chemical interactions also change. For example, seven nanometer hematite--a common iron oxide mineral--catalyzes the oxidation of manganese ions (Mn2+) one to two orders of magnitude faster than does a 37-nanometer hematite crystal, resulting in the rapid formation of the manganese oxide minerals that are important heavy metal sorbants in water and soils.
Thermodynamic considerations in the nano-range are just as critical to predicting whether a biogeochemical reaction will occur. In the smallest particles, surface energies can dominate and dictate which structure of a mineral will be stable. Solubility's of nanophases are also different than their larger counterparts. "But experiments have shown that nanoparticles may or may not be more soluble than larger particles," Hochella said.
How nanoparticles influence earth chemistry
An example of the impact of nanoparticles is how they nurture ocean-dwelling phytoplankton, which removes carbon dioxide from the atmosphere. Phytoplankton growth is limited by iron availability, the authors report, citing research by J. Wu, E. Boyle, W. Sunda, L.S. Wen, and B.A. Berquist in two articles from 2001 and 2007. Aupplied by rivers, glaciers, and atmospheric deposition, iron in the ocean is composed of nanocolloids, nanominerals, and mineral nanoparticles. Nanoscale reactions resulting in the formation of phytoplankton biominerals such as calcium carbonate are also important influences on oceanic and global carbon cycling.
Another example is the movement of harmful heavy metals in the Earth's critical zone. In ongoing research at the Clark Fork River Superfund Complex in Montana, Hochella's group discovered a nanocrystalline vernadite-like mineral (a manganese oxyhydroxide) involved in the movement of lead, arsenic, copper, and zinc hundred of miles in the river drainage basin. Radionuclides can also be moved, the review reports. Research by A.P. Novikov (2006) at one of the most contaminated nuclear sites in the world, a nuclear waste reprocessing plant in Mayak, Russia, has shown that plutonium has traveled in local groundwater, carried by nanoparticles of less than 15 nanometers.
In the atmosphere, nanoparticles impact heating and cooling. The characteristics of atmospheric nanoparticles are critical and are now being studied by a large number of scientists. One observation is that such particles act as water-drop growth centers, which is critical to cloud formation. The size and density of droplets dictate solar radiation scattering ability and cloud longevity, which influence average global temperatures.
The authors conclude that "the biogeochemical and ecological impacts of natural and synthetic nanomaterials are one of the fastest growing areas of research today, with not only vital scientific but also large environmental, economic, and political consequences."
 Learn more about the Hochella group. Learn more about the Hochella group.
 Read "Geoscientist solves living mystery, works to help environment." Read "Geoscientist solves living mystery, works to help environment."
|
|

